BB571061 — ChemDiv Building Block 3amino2,2dimethylpropan1ol
2,2-Dimethyl-1-propanol can be used in the synthesis of surfactant that stabilize reduced graphene oxide (rGO) dispersion. Biodiesel containing branched-chain esters prepared by the transesterification of vegetable oils with 2,2-dimethyl-1-propanol has been reported to show lower crystallization temperature when compared to methyl and ethyl.
Isomeria Quimicando

SOLUTION (10% CCl4 FOR 3800-1340, 10% CS2 FOR 1340-450 CM-1) Instrument: DOW KBr FOREPRISM-GRATING: Instrument parameters: BLAZED AT 3.5, 12.0, 20.0 MICRON AND CHANGED AT 5.0, 7.5, 14.9 MICRON: Path length: 0.011 CM, 0.011 CM SPECTRAL CONTAMINATION DUE TO CS2 AROUND 850 AND CCl4 AROUND 1550 CM-1 HAVE BEEN SUBTRACTED: Resolution: 2: Sampling.
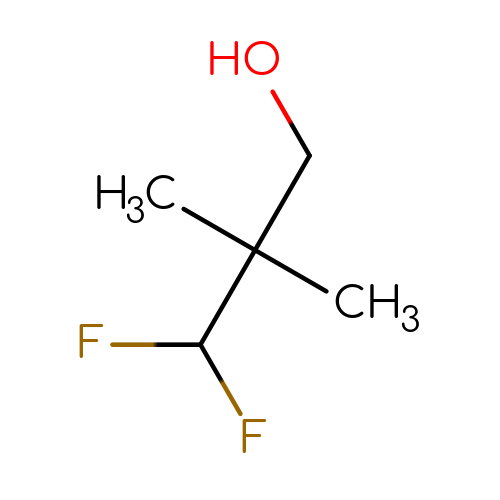
3,3difluoro2,2dimethylpropan1ol C5H10F2O 2098097573 MFCD29046500 CC(C)(CO)C(F)F
2,2-DIMETHYLPROPAN-1-OL; Neopentyl alcohol; tert-BUTYLCARBINOL; NEOPENTANOL; NEOAMYL ALCOHOL; 2,2-Dimethylpropanol; Propanol-1, 2,2-dimethyl-Neopentyl alcohol-O-D [1,1-2H2]Neopentyl alcohol. 2,2-Dimethyl-1,3-propanediol 2-Methyl-1,2-propanediol 3,3-Dimethyl-1-butanol Isobutyl alcohol-2-D1 2-Ammonio-2-methyl-1-propanol cation
A compound has two isomers (A) and (B) of formula C 5H10 O. Isomer (A) on treating with NaOH (aq

NIST/TRC Web Thermo Tables (WTT) NIST Standard Reference Subscription Database 3 - Professional Edition Version 2-2012-1-Pro This web application provides access to a collection of critically evaluated thermodynamic property data for pure compounds with a primary focus on organics. These data were generated through dynamic data analysis, as implemented in the NIST ThermoData Engine software.
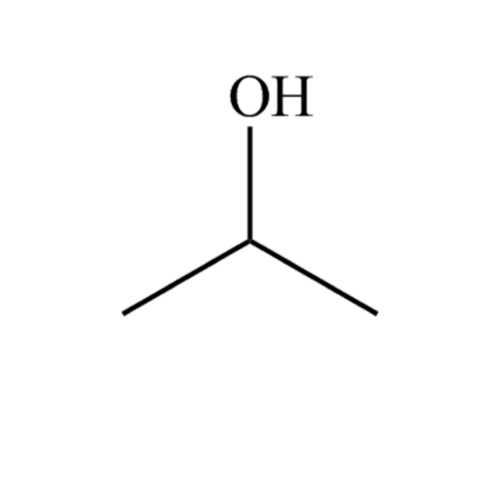
Propan2ol, HPLC RCI LABSCAN LIMITED (EN)
IUPAC Standard InChIKey: KPSSIOMAKSHJJG-UHFFFAOYSA-N Copy CAS Registry Number: 75-84-3 Chemical structure: This structure is also available as a 2d Mol file; Other names: tert-Butylcarbinol; Neoamyl alcohol; Neopentanol; Neopentyl alcohol; 2,2-Dimethyl-1-propanol; (CH3)3CCH2OH; 2,2-Dimethylpropyl alcohol; tert-Amyl alcohol; 2,2-Dimethylpropanol; 2-Methyl-isobutanol; 2,2,2-Trimethylethanol
Hydrogen NMR Example of 2,2Dimethyl1propanol YouTube

2,2-Dimethylpropan-1-ol;ethanol | C7H18O2 | CID 87562240 - structure, chemical names, physical and chemical properties, classification, patents, literature.
Chapter 8 Unit Test Paper Organic Chemistry Unit Test Paper Solutions for Class 10 Viraf

If the substance is covered by more than one CLH entry (e.g. disodium tetraborate EC no. 215-540-4, is covered by three harmonisations: 005-011-00-4; 005-011-01-1 and 005-011-02-9), CLH information cannot be displayed in the InfoCard as the difference between the CLH classifications requires manual interpretation or verification.
15260499223Amino1(5bromofuran2yl)2,2dimethylpropan1ol Ambeed

The 'Substance identity' section is calculated from substance identification information from all ECHA databases. The substance identifiers displayed in the InfoCard are the best available substance name, EC number, CAS number and/or the molecular and structural formulas. Some substance identifiers may have been claimed confidential, or may.
Which set of reagents will best convert 2,2dimethylpropan1ol (neopentyl alcohol) to

2,2-Dimethyl-1-propanol can be used in the synthesis of surfactant that stabilize reduced graphene oxide (rGO) dispersion. [1] Biodiesel containing branched-chain esters prepared by the transesterification of vegetable oils with 2,2-dimethyl-1-propanol has been reported to show lower crystallization temperature when compared to methyl and ethyl ester counterparts.
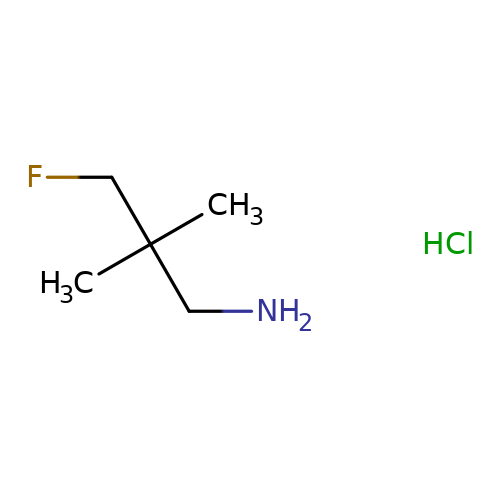
3Fluoro2,2dimethylpropan1amine hydrochloride 3DYID00022
IUPAC Standard InChIKey: KPSSIOMAKSHJJG-UHFFFAOYSA-N Copy CAS Registry Number: 75-84-3 Chemical structure: This structure is also available as a 2d Mol file; Other names: tert-Butylcarbinol; Neoamyl alcohol; Neopentanol; Neopentyl alcohol; 2,2-Dimethyl-1-propanol; (CH3)3CCH2OH; 2,2-Dimethylpropyl alcohol; tert-Amyl alcohol; 2,2-Dimethylpropanol; 2-Methyl-isobutanol; 2,2,2-Trimethylethanol
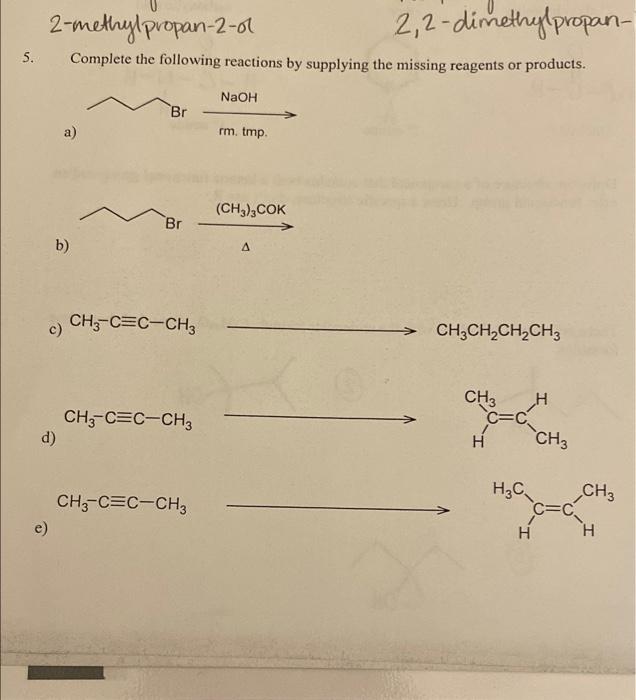
Solved 2methylpropan2ol 2,2dimethylpropan Complete the
Neopentyl alcohol is a compound with formula (CH 3) 3 CCH 2 OH. It is a colorless solid. The compound is one of the eight isomers of pentyl alcohol. Preparation and reactions. Neopentyl alcohol can be prepared from the hydroperoxide of diisobutylene. It can also be prepared by the reduction of trimethylacetic acid with lithium aluminium hydride.
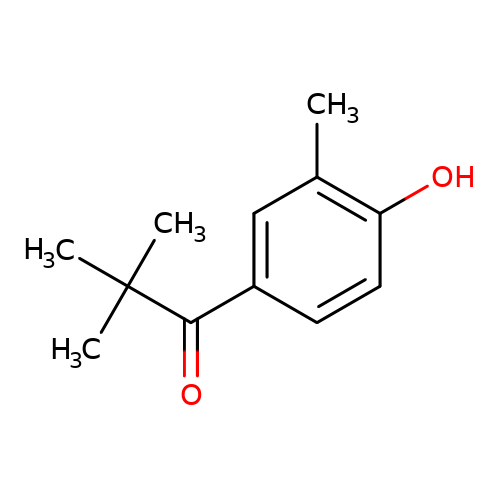
1(4Hydroxy3methylphenyl)2,2dimethylpropan1one 3DXCA56911
10.2.2 Artificial Pollution Sources. Amyl alcohols, such as neopentyl alcohol, are used as pharmaceutical preparations, flotation agents, and in organic synthesis (1). Therefore, neopentyl alcohol's production and use in these areas may result in its release to the environment through various waste streams (SRC).
A compound has two isomers (A) and (B) of formula C 5H10 O. Isomer (A) on treating with NaOH (aq

Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH 3) 2 CHCH 2 OH (sometimes represented as i-BuOH).This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and tert-butanol, all of which are important industrially.
Solved 2,2dimethylpropan1o Choose... (R)pentan2ol
2,2-DIMETHYLPROPAN-1-OL Catalog Id: MM75843 IUPAC: NEOPENTYLALCOHOL CAS Number: 75-84-3 More Information: Molecular Weight: 88.15 Formula: C5H12O SMILES: CC(C)(C)CO Preferred IUPAC Name: 2,2-DIMETHYLPROPAN-1-OL. Industriestrasse 1 CH-8890 Flums Switzerland Phone +41 81 740 58 30 .
HBr reacts fastest with Chemistry Questions

Now consider 2,2-dimethylpropan-1-ol. It is a primary alcohol and I was taught that it will not give a positive Lucas test.. So what you can theoretically say will be the only difference in say a Neopentyl alcohol and a 1-alkanol would simply be that after the super slow first step, the rest of the reaction will be much faster in the case of.
11Hbenzo[d]imidazol1yl2,2dimethylpropan1one

1-Amino-2,2-dimethylpropan-1-ol | C5H13NO | CID 3016233 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological.